A successful use of AFM is the result of the use of a high-quality microscope, the correct choice of an AFM probe and skillful operator work. One of the possible ways to verify whether the combination of these three prerequisites is available is the imaging of standard samples, which have been examined by other researchers and whose images are well described and characterized. Initially, getting atomic resolution in imaging of mica in contact mode was considered as proof the AFM microscope performance. This task was not difficult, as was the case with the visualization of the chain order (spacing of 0.56nnm) on the surface of rubbed polytetrafluoroethylene layers. More difficult is the recording of atomic-scale patterns of HOPG in tapping mode (see High-Resolution Imaging), therefore the nanometer-scale structures are often used for demonstration of the microscope capabilities.
| Fig.1. Images courtesy of Prof. D. A. Ivanov (Free University of Brussels). Samples courtesy of Prof. G. Ungar (Sheffield University, England) |
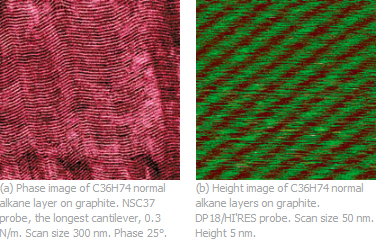 |
For example, layers of normal alkanes (CnH2n+2) on HOPG (Fig. 1) are built of lamellar structures whose alignment mimics the 3-fold symmetry of the substrate. The different size and mobility of the -CH3 end groups compared to the chain groups -CH2- is responsible for the stripped pattern observed in the AFM images recorded in tapping mode. The spacing is defined by the length of the alkane molecules and varies from 1.4nm in C18H38 to 49nm in C390H782. Imaging of C60H122 alkane layers (the spacing is ~7.5nm) is routinely used for demonstration of high-resolution of commercial AFM microscopes.
Imaging of films of block copolymers such as SBS is also common for routine checks of the microscopes or scanners. These samples have regular patterns with average spacing in the 20-40nm range and do not bring problems for imaging. The patterns are clearly resolved in height and phase images when the blocks are plastic and rubbery. The observation of the high-contrast height and phase images is related to differences in the mechanical properties of these blocks. In such cases, the assignment of the height images to surface topography can be misleading because the contrast of height images can be caused by a cross-talk with the phase pattern that reflects a difference of AFM tip interactions with dissimilar blocks. In this case, a minimization of the tip-sample force by choosing soft AFM probes such as HQ:NSC19 (stiffness below 1N/m), is needed for recording true sample topography.
The samples mentioned above have relatively flat surfaces and demand less attention to optimization of feedback gains and scanning rate. The situation is different when examining such samples as latex arrays (see Latex systems) or microporous surface like that of Celgard membrane made of isotactic polypropylene. The alternation of nanofibrils, which are separated by nanoscale cavities, and elevated lamellar regions characterizes morphology of this sample. It takes some effort to get tapping mode images of this material with nm-scale resolved features without disturbing the interfibrillar cavities. Silicon AFM probes with stiffness ~5N/m (HQ:NSC14) are the right choice for such topography studies. Non-destructive imaging of this sample in contact mode is only possible under water.
Three of the described samples: mica, HOPG and commercial Celgard film can be studied with AFM without any preparation. Mica and HOPG are layered materials and their fresh surfaces are prepared by cleaving with a sticky tape. Other samples need some kind of preparation. Hot rubbing of PTFE on different substrates is used for making sheets of oriented polymer chains. Spin casting of a block copolymer solution on a substrate leads to the formation of films of different thicknesses in which microphase separation layer originate during solvent evaporation. Annealing of these samples at high temperature or in solvent vapors improved this order and this process can be monitored by AFM. Other deposition methods such as dipping, droplet deposition and the use of Langmuir-Blodgett trough are also employed for preparation of single macromolecules on different substrates and different self-assembled layers. A proper choice of solution concentration, a nature of substrate and annealing procedure are helpful for preparation of the optimal sample.
More elaborative is the preparation of polymer bulk materials for AFM studies of their morphology. This is usually done with the help of ultramicrotome. A choice of a diamond knife, cutting speed and temperature are crucial for preparation of smooth surfaces with minimal morphology disturbance. This can be achieved and lamellar structures with dimensions in the 10-20nm range can be observed on microtomed surfaces. Examples of the AFM studies of complex polymer materials on samples prepared with the ultramicrotome are given in Heterogeneous Polymer Systems.
Further reading
Robust samples
Soft, fragile and near-liquid samples
